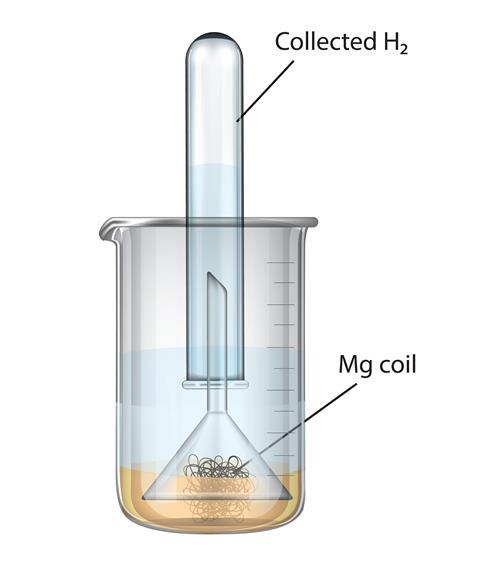
What happens when Magnesium oxide is dissolved in water? Write a word equation for this process. Name - Brainly.in
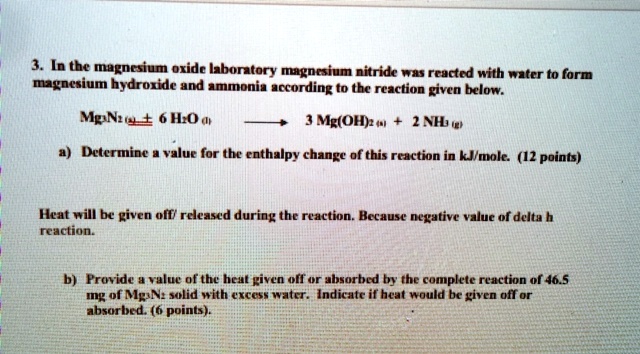
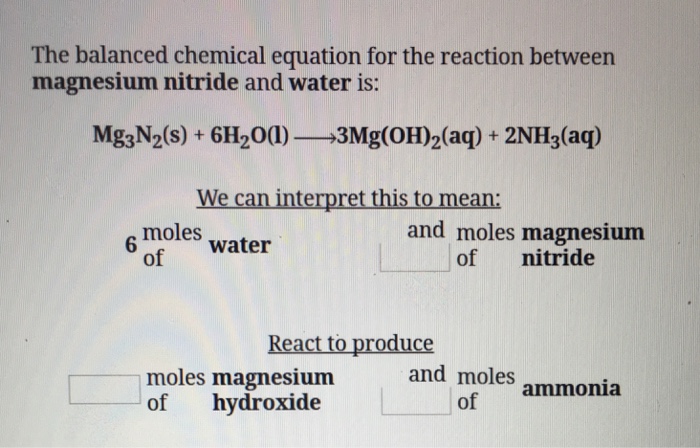
SOLVED: In the magnesium oxide babonatory magnoium nitride ras reacted with water to form ntsium hydroxide and nnm according to the reaction given below. MgNiel + 6 H:O a Mg(OH) ( 2

Question Video: Ordering the Reactions of Magnesium Metal with Varying Concentrations of Nitric Acid | Nagwa

Question Video: Balancing a Chemical Equation for the Reaction between Magnesium Nitride and Water | Nagwa
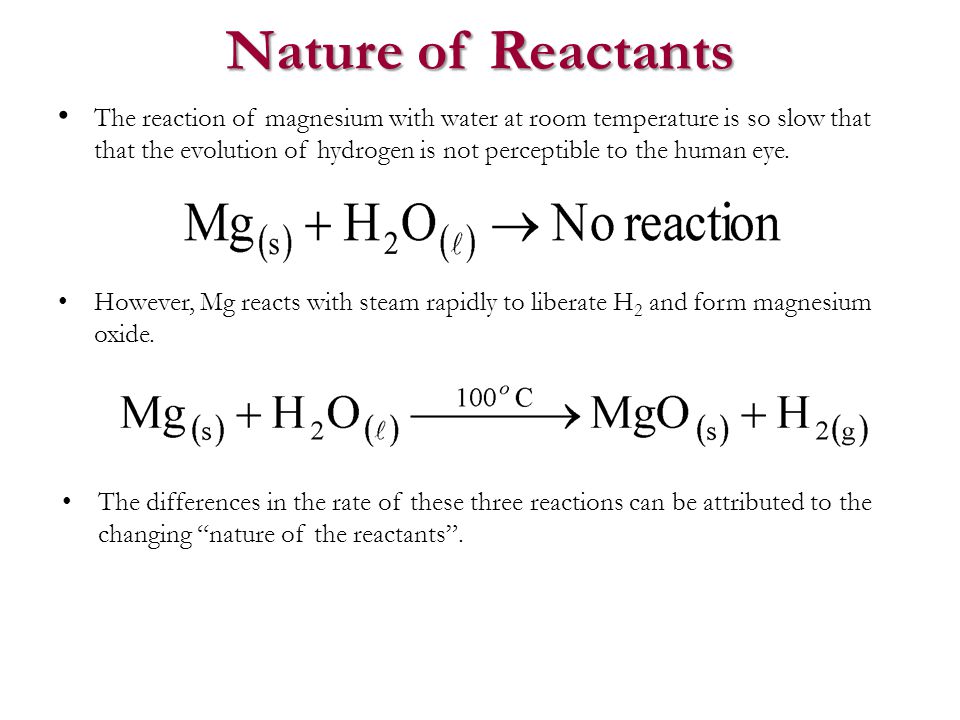
why calcium and magnesium floats on water - Science - Metals and Non-metals - 13061069 | Meritnation.com