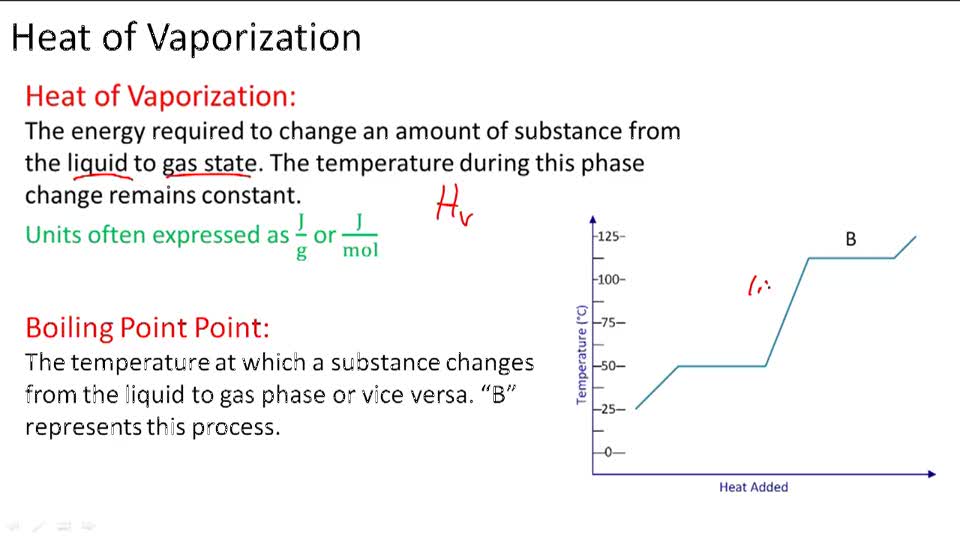
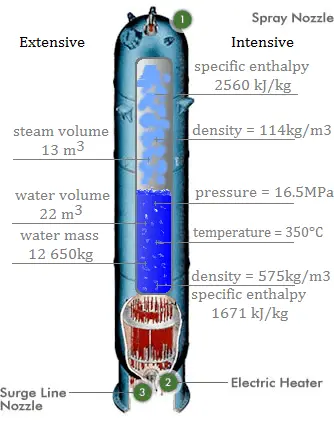
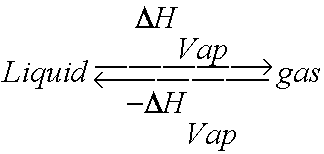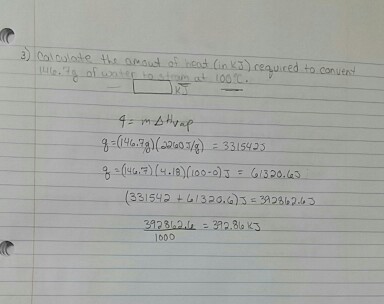SOLVED: The Clausis-Clapeyron equation relates vapor pressure; enthalpy of vaporization, and temperature: AHL In p The equation can als0 be expressed in two-point form; AH, Decide which form to use,and solve the

The enthalpy of vaporization of water at 100^o C is 40.63 KJ mol^-1 . The value Δ E for this process would be:

SOLVED:For mercury, the enthalpy of vaporization is 58.51 kJ/mol and the entropy of vaporization is 92.92 J/K ? mol. What is the normal boiling point of mercury?
Standard enthalpy of vaporisation ΔvapH° for water at 100°C is 40.66 kJ mol^-1. - Sarthaks eConnect | Largest Online Education Community
88. The values for delta H vap.and delta S vap. for ethanol are respectively 38.594 kJ/mol and 109.8 J/K. What will be the boiling point of ethanol ?

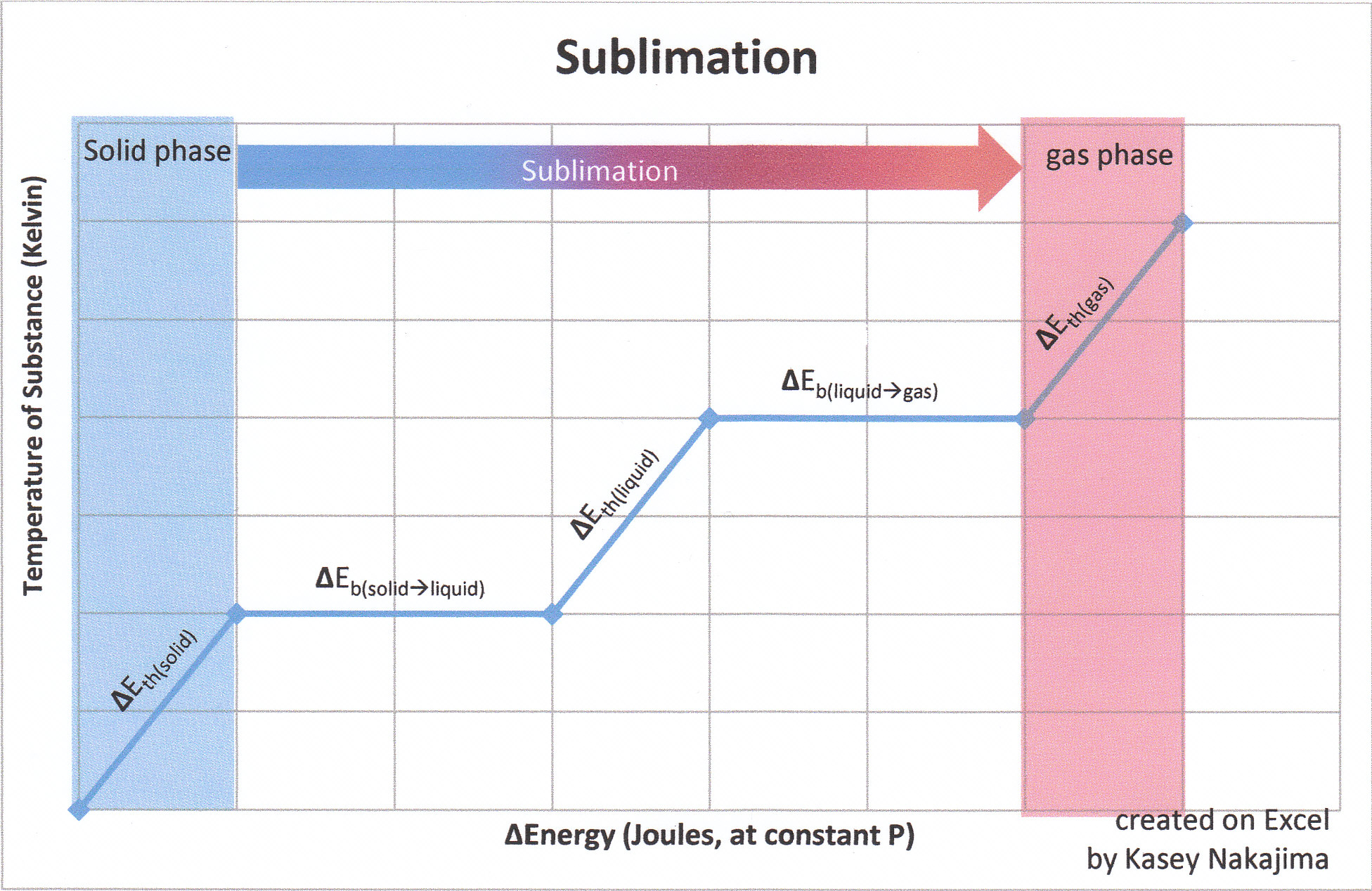

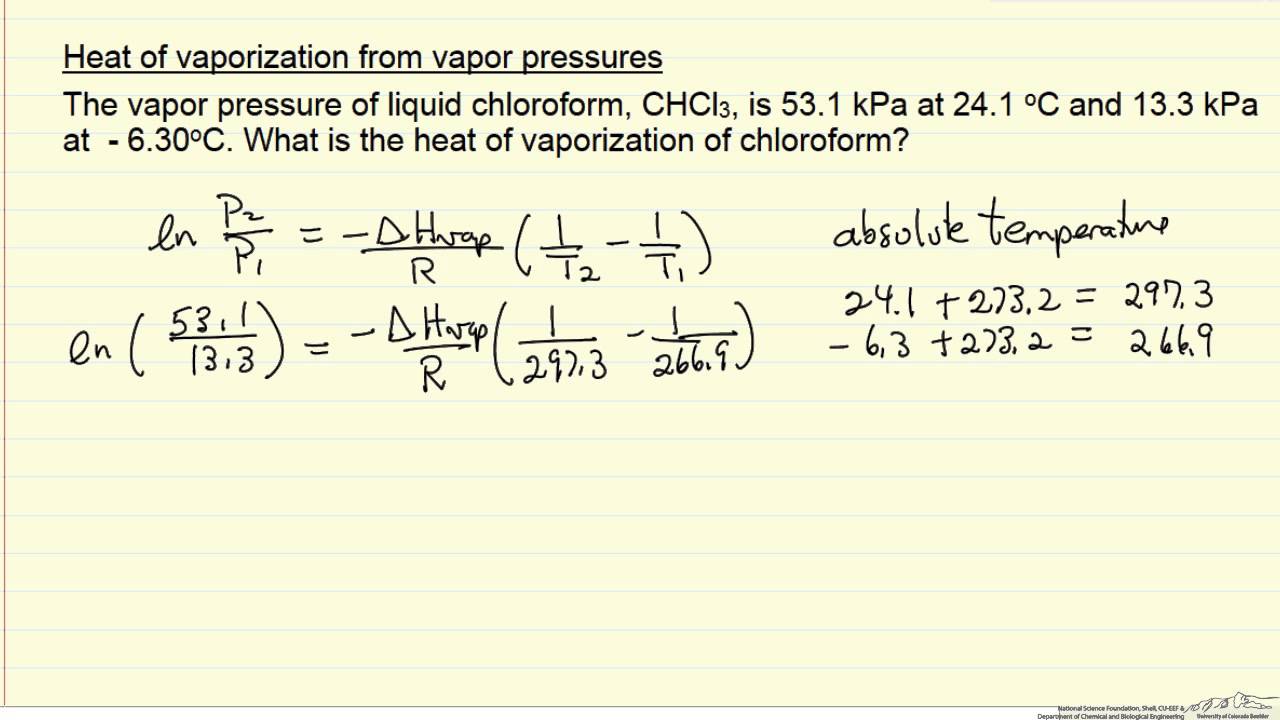
-438.png)