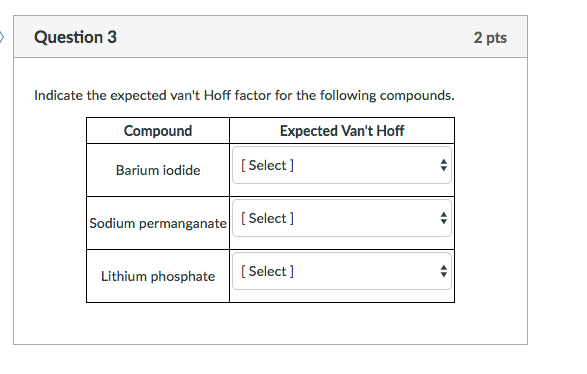
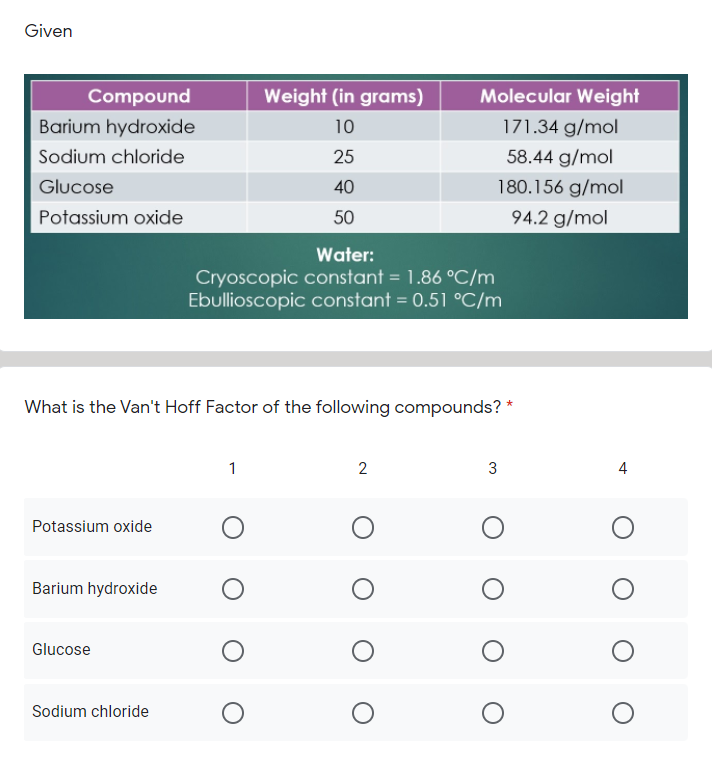
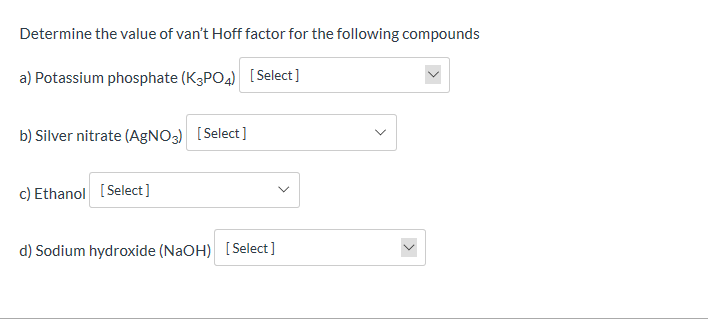
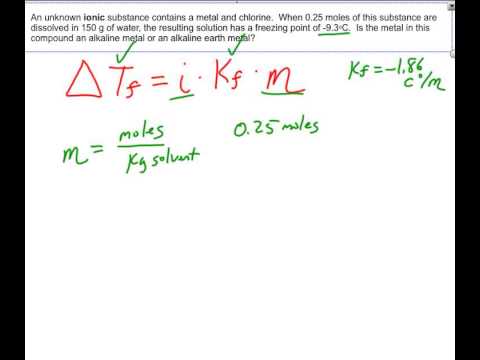
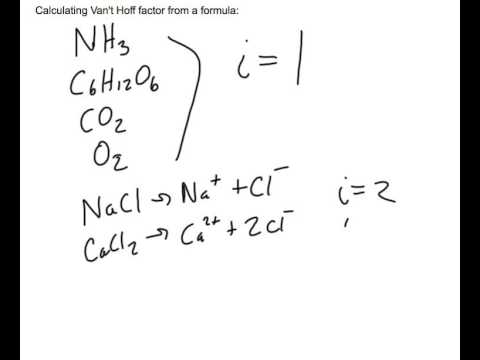
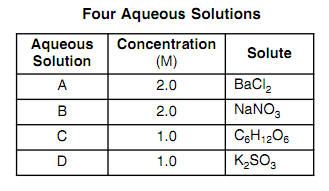
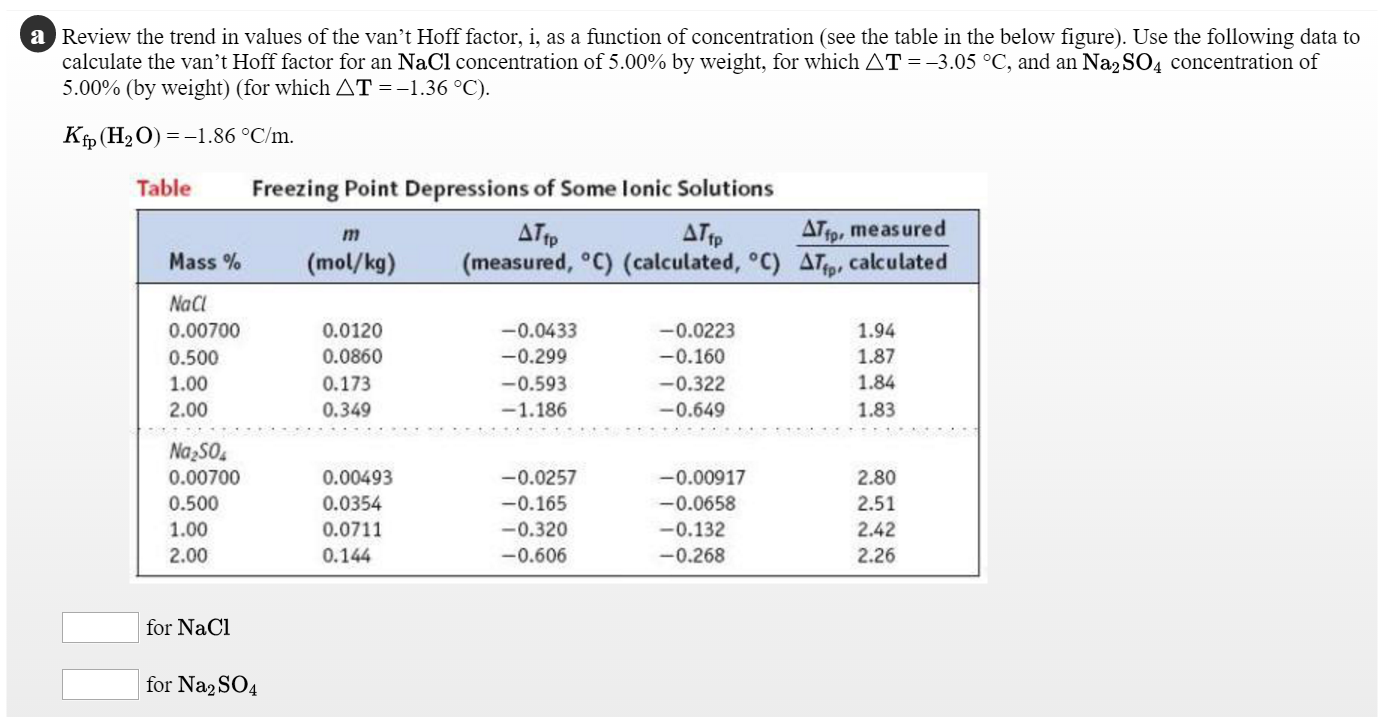
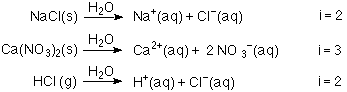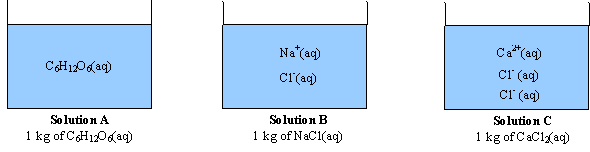OneClass: Indicate the expected van't Hoff factor for the following compounds. Sodium carbonate Cesiu...

OneClass: Calculate the molality and van't Hoff factor (i) for the following aqueous solution: 0.925 ...

Predicting Van't Hoff (i) Factors: Colligative Properties | Teaching chemistry, Chemistry worksheets, Teaching middle school science

Van't Hoff factor as a function of concentration (calculated according... | Download Scientific Diagram
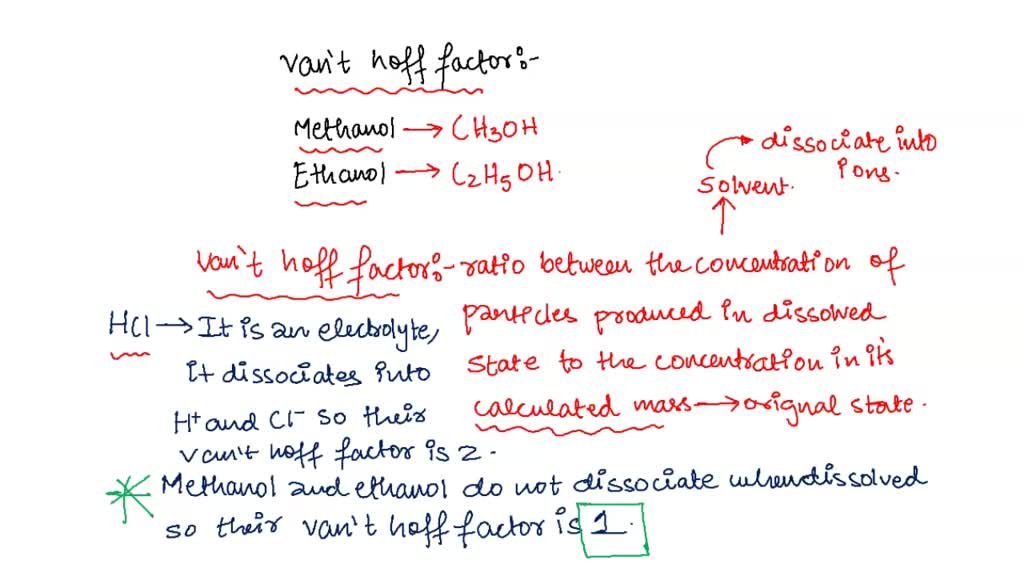
SOLVED: Question 2 0.5 pts What is the van't Hoff factor for methanol? Question 3 0.5 pts What is the van't Hoff factor for ethanol?